The EPTRI management structure is designed to:
- Ensure that the project works as an integrated entity between the different WPs
- Provide timely and efficient scientific, financial and administrative coordination of the project
- Guarantee a transparent process for decision making and conflict resolution
- Implement high-quality execution of the project.
It will include the following bodies, as shown in the figure below:
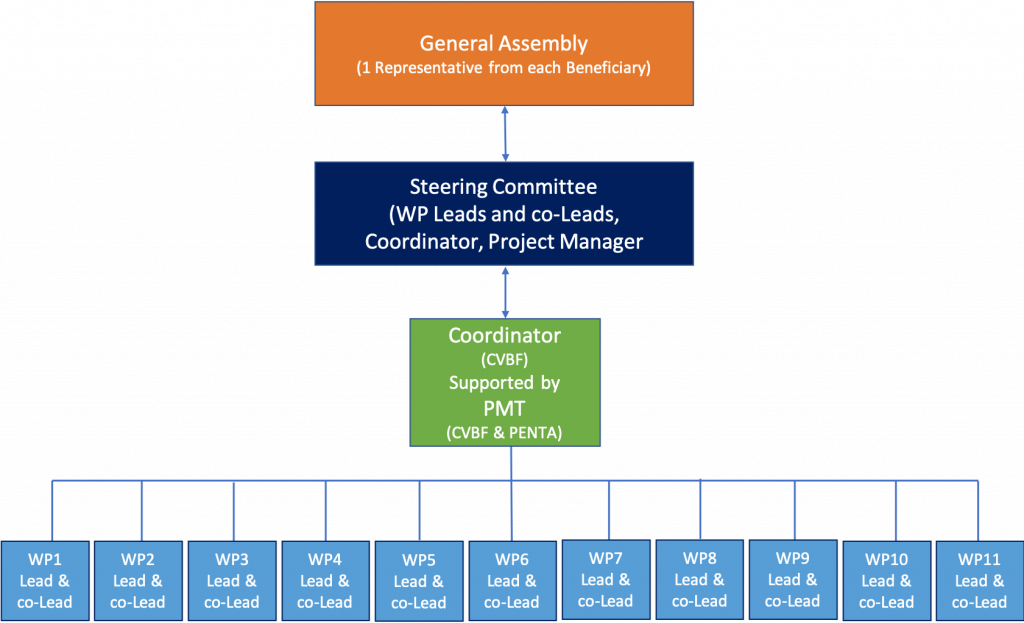
The Project Coordinator (PC) paces and coordinates the activities, ensures timely release of deliverables and supervises the budget management while interacting with the EU Commission. The Coordinator activities are overseen by the governance structure of the project - the Steering Committee.
The General Assembly (GA) will be the governance body for the project. It is composed of the Project Beneficiaries and its role consists of making decisions on the governance and management of the project.
The Steering Committee (SC) is composed of the project coordinator, the work package leaders and the co-leaders that will be appointed at the beginning of the project. Its role will consist of driving and coordinating the work package activities as well as preparing the feasibility plan and the CDR. It will define the organisation and the operative procedures of each operational platform.
A Project Manager (PM), in the person of the Work Package 1 Leader, is envisaged to ensure a technical coordination of persons, structures and activities, complementing the coordination actions described above.
The Project Management Team (PMT) is led by the Project Manager, it will follow-up activities and monitor compliance with the work plan, planned resources and time schedule.
The Work Package Leader (WPL) will ensure permanent follow-up of activities, in direct contact with involved participants and will report on a quarterly basis to the PMT.
The Advisory Board (AB) is a multi-disciplinary board composed of 4 internal and 3 external members of the EPTRI consortium with a one-year mandate, appointed by the EPTRI Steering Committee.
The Board composition ensures the representation of different and complementary expertise:
- Paediatric research methodology – Prof. Adriana Ceci (Chair)
- Toxicology/Pre-clinical – Dr. Giovanni Migliaccio
- Biomarkers/Biosamples – Prof. Oscar Della Pasqua
- Developmental Pharmacology – Prof. Catherine Knibbe
- Formulation/Devices – Prof. Catherine Tuleu
- Data-science/Bioinformatics – Prof. Graziano Pesole
- HTA/Ethics/Regulatory – Dr. Dimitrios Athanasiou (Patients’ representative).
The AB is in charge to assess and review Feasibility Studies proposals received from WPs/Tasks Leaders during the third phase of the EPTRI project. These studies could function as a test of EPTRI capability to deliver services and provide access to samples and facilities that could be needed to conduct paediatric research in the areas of competence of EPTRI according to the users’ needs.
Moreover, it provides advice in the definition of the service request workflow. The points on which the opinion should be articulated include some key questions:
- a) the proposal meets EPTRI mission and abilities;
- b) the proposal is likely to be completed by the end of ID-EPTRI project;
- c) the scientific contents are valuable;
- d) services and facilities within EPTRI are available to perform the work;
AB will be also in charge to request the EPTRI Ethical Advisory Board (EAB) ’s advice for any of the proposed feasibility study and to assess how to involve paediatric patients in the feasibility study procedure.
